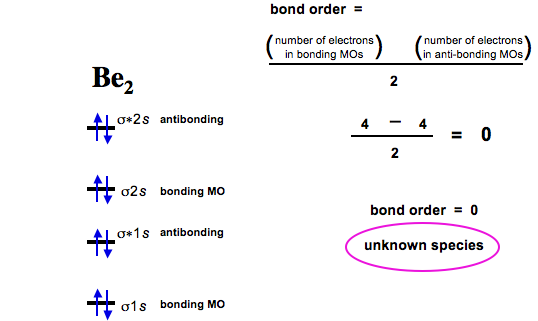
Jun Diberyllium is thought to be non-existant. This website explains how diberyllium cannot exist in brief and simple language. Molecular Orbital Diagram for Beryllium Dimer ( Be) Fill from the bottom. Bonding Order ismeaning it does.
Chemistry › Electron Configurationsocratic. Explanation: According to MO theory, the bond order is and Be has two bonding electrons with one antibonding electron. Is bediamagnetic?

AnswersDrive answersdrive. MOT its bond order comes out to be zero. Hence beis neither diamagnetic nor paramagnetic. Mar Write the molecular orbital electronic configuration of Bemolecule.
So, this is diamagnetic. Bebond order contactpakistan. Understand the difference between diamagnetism and paramagnetism, and describe.
Dec Diamagnetic : a material which become magnetic only in the presence of an external magnetic field. The magnetic permeability is under 1. Identify each molecule as diamagnetic or paramagnetic. Bond-order and Magnetic Behavior of Diatomic Species. WJCE-5-4-2article.
Since, there is one unpaired electron in He– hence it should be paramagnetic. The MO configuration for Beis given below, and shown in Fig. Paramagnetic Stable Paramagnetic Stable Fig. Atomic numbers: H= He= Li=.
Practice Questions - SMC Faculty Home Pages homepage. His more stable because it is diamagnetic, whereas Beis paramagnetic. Karl-Heinz Bennemann, John B. Bond order value suggests that the molecule is unstable and diamagnetic. Since it has one unpaired electron, hence it is paramagnetic in nature.
Assuming 2s-2p mixing is NOT operative,the paramagnetic species among the following is. Are they diamagnetic or paramagnetic ? Do you expect these molecules to exist in the gas phase?
A molecule in which all the electrons are paire is called diamagnetic while molecule which has one or more unpaired electron is called paramagnetic. Generate a molecular orbital diagram for Beand remove an electron from the highest.
Also Cis diamagnetic. Bookshelves › Magnetic_Behavio. B calculate the bond order. I need help drawing this molecular orbital.
Using your MO energy level diagram in (a), fill in information about Beand Be. This shows no unpaired electrons, so it predicts that Ois diamagnetic. Liand Be, and also.
This ability of MO theory to explain the paramagnetism of Ogave it credibility as a major. The diagram for H He Li Be, B C and N2. Unpaired electron, PARAMAGNETIC.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.