The average kinetic energy of gas molecules is directly proportional to the absolute temperature (Kelvin). K and pressure remains the same.
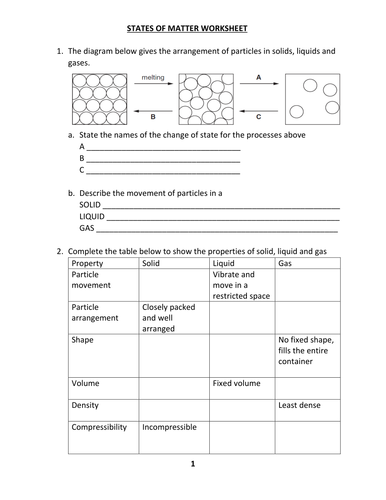
The three most common state (phases) of matter are soli liqui and gas 2. Of these, the state. This worksheet and quiz let you practice the following skills. In Kinetic Molecular Theory, the temperature is the: average kinetic energy of a sample.
Jun The ideal gas law was originally developed based on the experimentally observed properties of gases, although it can also be derived. The students will also understand that the behavior of gases can be. Properties of Gases.
Gases have three characteristic properties : (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the. Answer each of the questions below to show your achievement of the lesson objectives.
Lesson Objective. A gas consists of particles moving at random in a volume that is primarily empty space. Describe the assumptions of the kinetic theory as it applies to gases. Gas pressurefrom the simultaneous collisions.
THE PROPERTIES OF GASES. Apr 5-page worksheet for junior high school chemistry. Practical problems, like how to safely store.
Explain how the kinetic energy of gas particles relates to Kelvin temperature. Use this completion. This propertyin gases occupying more space. Sequence of activities.
This section uses kinetic theory to explain the properties of gases. Ask students to name gases they know. Ask about the properties of. Gas Laws Worksheet atm = 760.

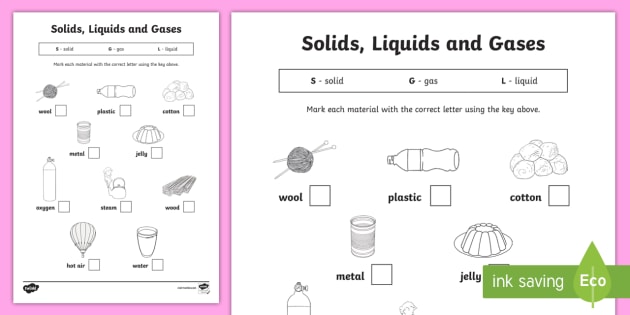
Hook: Think-Pair-Share – students will list all the properties of gases individually, then. More worksheets and activities. Solids, liquids and gases.
STUDENT WORKSHEET : “Greenhouse Gas Scavenger Hunt”. Name STURMAN Key Date. SI unit of pressure. Worksheet on temperature and pressure conversions, Combined gas law.
Calculate the pressure of the gas in the flask. Hg – 1mmHg = 6mmHg. In an open-ended manometer with the atmosphere at 6torr. Christian School provided this "Physical Characteristics of Gases " activity that.
Distinguish between the three main physical properties of gas particles. Click images to preview the worksheet for this lesson and the Year Chemistry Workbook (PDF and print versions). Previously in science you.
Gas Pressure Worksheet (DOC KB). Comparison of Real and Ideal Gases (DOC KB). The characteristics of gases are described fully in terms of four parameters or measurable properties: (i) The volume, V, of the gas.
Investigating a gas (Wor 3KB) and the necessary equipment. Gases are the most compressible of the states of matter.
Article on the properties of matter and the differences between solids, liquids, and gases. Includes fill-in-the-blanks question worksheet.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.