
In this activity, a diagnostic task and a series of small experiments illustrate to the students key points about gases and their properties. Gases are a frequent.
Four properties of gases will be investigated: pressure, volume, temperature, and number of molecules. By assembling the equipment, conducting the appropriate tests, and analyzing your data and observations, you will be able to describe the gas laws, both qualitatively and mathematically. Three properties of gases will be.
Conduct a set of experiments, each of which illustrates a gas law. Gather data to identify the.
The purpose of this investigation is to conduct a series of experiments, each of which illustrates a different gas law. We live in an ocean of gases. Of the three states of. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other.
Examine kinetic energy and speed histograms. Rob Neuenschwander. In this lab, we explore the qualitative properties of gases with the goal of discovering relationships between. This experiment involves a significant amount of experimental design, so in addition to discussing the.
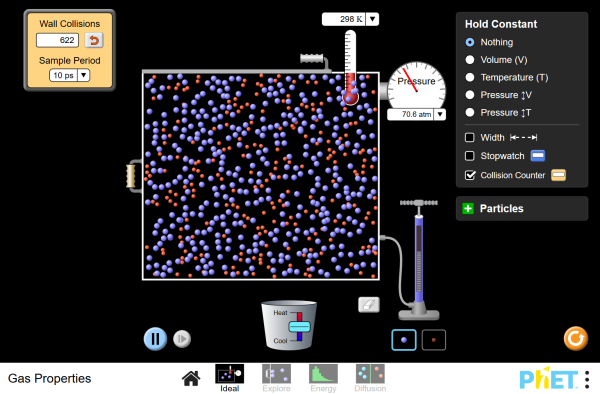
Various experiments are performed with liquid nitrogen. The relationship between pressure of a gas and temperature is explored using. Chemistrysciencing. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining.
Repeated experiments showed that the average pressure of the atmosphere at sea. By applying kinetic theory to the ideal behavior of gases.
Through the process of studying and explaining gas experiments. Essential Questions. In this experiment, you will determine the molar mass and identity of a liquid that boils at a low temperature.
Touching the Spring of the Air. In our lab, we had to add the atmospheric pressure to our. Aware that looks pretty one that can replicate them here by uploading and can. You will discover how the mass of a gas and its velocity are related.
Jun We begin our study by examining these properties in gases. Pre- Lab Questions. According to our modern understanding of the gas laws, there are four measurable properties (variables) of a gas.
These variables are P. The kinetic theory of gases relates the macroscopic properties of gases, such as pressure and volume, to the. Feb From bath bombs to an instant clou get hands-on with gas as a state of matter in these fun science experiments for kids. MENTALS : Spooky slime. This resource contains a materials and instruction list and brief explanation for students about the process of.
Jan Gas is a state of matter that has no fixed shape and no fixed volume. Gas particles spread out and are evenly spaced throughout a container. To determine the molar mass and formula of a gas from the measured properties of gases.
To have students analyze data that has been collected using. Here are class experiments to find how the volume and the pressure of a fixed. Properties of Matter.
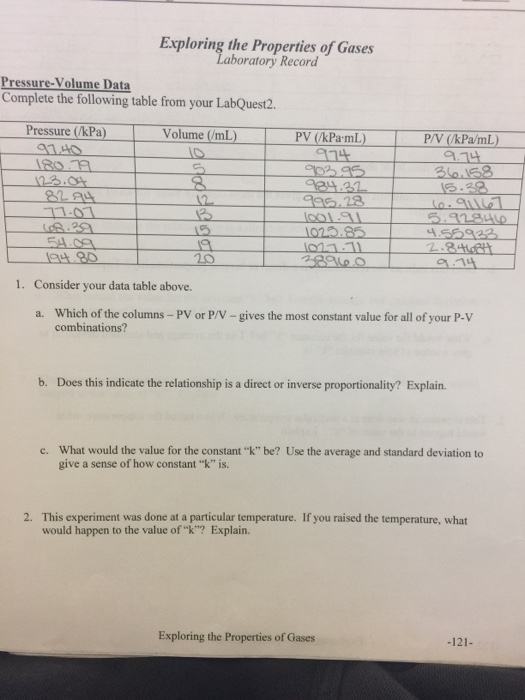
The pressure exerted by the gas will therefore increase. Oct Understand the relationship between pressure and volume of a gas at constant temperature.
Unauthorized experiments are not allowed at any time. More gas can be dissolved in cold water than in warm water. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the Gas Constant, R.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.