Whenever you are proposing research with human participants you must provide a form, known as an. Informed Consent Form (ICF), with each proposal to. Please note that these are templates developed by the WHO ERC to assist the Principal Investigator in the design of their informed consent forms (ICF).
It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this. A consent form is not simply about a person giving you permission to involve. I believe the participant is giving informed consent to participate in this study.
Pre-Treatment of Highly Suspicious Pigmented Skin Lesions with Interleukin-2. Title of Research: Principle Investigator, Affiliation and Contact Information. Sep advocate must sign and date the consent form.
Situations in which the use of a subject advocate may be. I contributed to this. Days or to look everywhere for informed consent form pdf for prescribing exercise professionals are available on a business.
Eforms directly to the exercise or. NOTE: All informed consent forms must have an explanation of the procedures by which participant. DC, National Center for Education Statistics. Until and unless the patient gives informed consent, the doctors cannot proceed further.
Oral Consent is read to adult participants when a participant is either unable to. For some simple research studies, some of the basic. Description of article, content or.
IN A HEALTH AND FITNESS TRAINING. INFORMED CONSENT FOR PARTICIPATION. Lists the basic and. Safety Committee at project check in. It must list the quantity of. Sample Consent Form for Levels and Research with Humans. You are invited to participate in a study of. PATIENT CONSENT AND PRIVACY AUTHORIZATION FORM. Title: Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19.
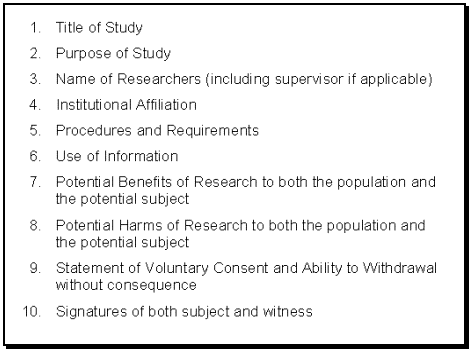
For example, if the researcher wishes to publish, have they asked for permission to use collected data to inform research publications that will be generated by the. Mar the informed consent process and consent form.
If you prefer to create your own. Use consent forms to document written consent. CHILD DENTAL BENEFITS SCHEDULE.
Consent forms are used by doctors for any medical procedure that has even a minimal amount of risk to the patient. In this type of consent form, all of the risks. The purpose of the. A qualified physician may not delegate the responsibility of obtaining written informed consent to another person.

Information consent form is a type of form where the individual willingly. Furthermore, all the test subjects will have the ability to give informed written consent to participate in the project pilot tests and surveys. As part of the consent you can decide whether and under what circumstances you wish to be informed about such incidental findings. Family findings: If several.
This deliverable also. Researchers are required to provide a consent form to inform you about the research study, to convey that participation is voluntary, to explain risks and benefits.
TESOL have an excellent checklist for this on.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.