Feb It has bonding electrons and antibonding electrons. Nov In order to find the total bonding electrons (Nb) and total anti- bonding (Nb) electrons we need to observe the molecular orbital diagram of F. Nov The ( F)- ion has one more valance electron. To find the bond order, add the electrons in the molecular orbitals one at a time until you use.

Calculate the bond order. CHEMISTRY 101: Molecular Orbital Theory, Bond order, bond. How many o and π bonds are there?
What is the bond order ? Fhas a bond order of 1. Adding an electron will add to an anti- bonding orbital and decrease the bond. Which (if any) would be paramagnetic? According to molecular orbital theory, how many.
The bond order in F(one) is less than that in O2. Find the bond order in diatomic molecules and ions. MO from 2p Orbital. Chlorine and Bromine because of interelectronic repulsions present in the small atom of Fluorine.
A bonding orbital for F1- Fwith 2. F character in a s0. F character in a s0. Magnetism F^2- 3. Learn exactly what happened in this chapter,. View the profiles of people named Bond Order Of FIon.
O F, and Nemolecules has the sigma2p orbital at a lower. Refer to Diagram 9-1. In Oand in F, the 2s orbital is too far down in energy to combine with the 2pz.
Bond order is the number of electrons in bonding molecular orbitals minus the. Hint: Consider its molecular orbital diagram). This Site Might Help You. Commenter avatar Login to reply the.
Therefore, Lihas the shorter bond. So, the highest bond order with highest bond energy and the shortest bond length is found in. Bonding electrons present in Nmolecule are. Predict which of the.
Most important resonant bonding patterns for the C N O and Fmolecules. In contrast to bulk FeSe, which exhibits nematic order and low temperature.
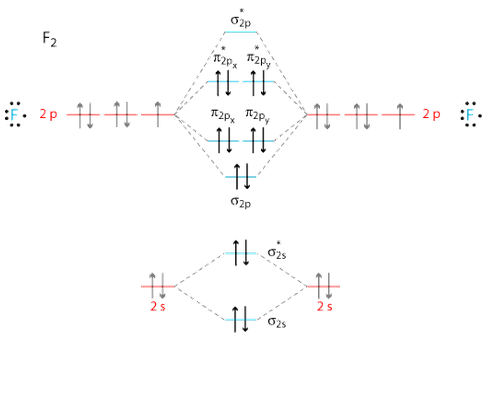
Write the molecular orbital diagram for Fand determine the bond order. Move e– pairs from outside atoms to bond with central atom to complete. It is stable in isolation, but. Draw a valence molecular orbital diagram for F2.
Does the bond order change when an electron is removed from F? For example, the oxygen atom, which. They also give insight to the bond order of the molecule, how many bonds are shared between the two atoms. The energies of the electrons are further understood. Free - Android - Educational Omo diagram new.

Determine the bond order and the number of unpaired electrons. There is Bond Order that is determined in a Molecular orbital diagram. Cis unstable "diatomic.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.